- info@h2-aqua.com
Facts and Studies
- Home
- Facts and Studies
Categories
Hydrogen Water Generators
HydroLUX H2
View ProductContact Us
Email Address
info@h2-aqua.com
Hydrogen: An Emerging Medical Gas
Introduction
 Molecular hydrogen (i.e. H2 gas) is gaining significant attention from academic researchers, medical doctors, and physicians around the world for its recently reported therapeutic potential [1]. One of the earliest publications on hydrogen as a medical gas was in 1975, by Dole and colleagues from Baylor University and Texas A&M [2]. They reported in the journal Science that hyperbaric (8 atm) hydrogen therapy was effective at reducing melanoma tumors in mice. However, the interest in hydrogen therapy only recently began after 2007, when it was demonstrated that administration of hydrogen gas via inhalation (at levels below the flammability limit of 4.6%) or ingestion of an aqueous-solution containing dissolved hydrogen, could also exert therapeutic biological effects [3]. These findings suggest hydrogen has immediate medical and clinical applications [4].
Molecular hydrogen (i.e. H2 gas) is gaining significant attention from academic researchers, medical doctors, and physicians around the world for its recently reported therapeutic potential [1]. One of the earliest publications on hydrogen as a medical gas was in 1975, by Dole and colleagues from Baylor University and Texas A&M [2]. They reported in the journal Science that hyperbaric (8 atm) hydrogen therapy was effective at reducing melanoma tumors in mice. However, the interest in hydrogen therapy only recently began after 2007, when it was demonstrated that administration of hydrogen gas via inhalation (at levels below the flammability limit of 4.6%) or ingestion of an aqueous-solution containing dissolved hydrogen, could also exert therapeutic biological effects [3]. These findings suggest hydrogen has immediate medical and clinical applications [4].
This MHF article does not discuss negative hydrogen ions, (hydride, H-), pH (e.g. alkaline water), microclustered water, or other topics that are subject to pseudoscientific claims.
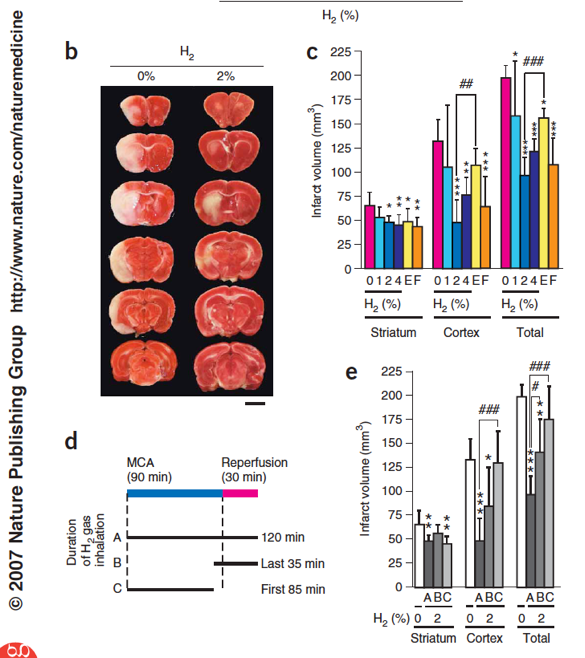 In 2007, Dr. Ohta’s team reported in Nature Medicine [3] that inhalation of 2-4% hydrogen gas significantly reduced the cerebral infarct volumes in a rat model of ischemia-reperfusion injury induced by middle cerebral artery occlusion. Hydrogen was more effective than edaravone, an approved clinical drug for cerebral infarction, but with no toxic effects (See figure on left). The authors further demonstrated that dissolved hydrogen in the media of cultured cells, at biologically relevant concentrations, reduces the level of toxic hydroxyl radicals (*OH), but does not react with other physiologically important reactive oxygen species (e.g. superoxide, nitric oxide, hydrogen peroxide).
In 2007, Dr. Ohta’s team reported in Nature Medicine [3] that inhalation of 2-4% hydrogen gas significantly reduced the cerebral infarct volumes in a rat model of ischemia-reperfusion injury induced by middle cerebral artery occlusion. Hydrogen was more effective than edaravone, an approved clinical drug for cerebral infarction, but with no toxic effects (See figure on left). The authors further demonstrated that dissolved hydrogen in the media of cultured cells, at biologically relevant concentrations, reduces the level of toxic hydroxyl radicals (*OH), but does not react with other physiologically important reactive oxygen species (e.g. superoxide, nitric oxide, hydrogen peroxide).
This biomedical research on hydrogen is still in its infancy with only around 1000 articles and 1,600 researchers, but these publications and researchers suggest that hydrogen has therapeutic potential in over 170 different human and animal disease models, and in essentially every organ of the human body [5]. Hydrogen appears to provide these benefits via modulating signal transduction, protein phosphorylation, and gene expressions (See section Pharmacodynamics) [4].
The idea of therapeutic gaseous molecules is not a new concept. For example, carbon monoxide (CO), hydrogen sulfide (H2S), and of course nitric oxide (NO*), which was initially ridiculed by skeptics, but later was subject to a Nobel Prize, are all biologically active gases [6]. However, it may still be difficult to believe that H2 can exert any biological effect, because in contrast to these other gases, hydrogen is a non-radical, non-reactive, non-polar, highly diffusible neutral gas, thus it is unlikely to have specific binding sites, or interact with specificity on a specific receptor [7].
From an evolutionary perspective it may not be strange that hydrogen exerts a biological effect [8]. In addition to its role in the origins of the universe, hydrogen was also involved in the genesis of life and played an active role in the evolution of eukaryotes [9]. Over the millions of years of evolution, plants and animals have developed a mutualistic relationship with hydrogen-producing bacteria resulting in basal levels of molecular hydrogen in eukaryotic systems. This constant exposure to molecular hydrogen may have conserved the original targets of hydrogen, as can be extrapolated by genetic remnants of hydrogenase enzymes in higher eukaryotes. Alternatively, but not exclusively, eukaryotes may have developed sensitivity to molecular hydrogen over the millions of years of evolution [7, 10].
METHODS OF ADMINISTRATION
Molecular hydrogen can be administered via inhalation [11], ingestion of solubilized (dissolved) hydrogen-rich solutions (e.g. water, flavored beverages, etc.) [12], hydrogen-rich hemodialysis solution [13], intravenous injection of hydrogen-rich saline [14], topical administration of hydrogen-rich media (e.g. bath, shower, and creams) [15], hyperbaric treatment [2], ingestion of hydrogen-producing material upon reaction with gastric acid [15], ingestion of non-digestible carbohydrates as prebiotic to hydrogen-producing intestinal bacteria [16], rectal insufflation [17], and other methods. [15].
PHARMACOKINETICS
Hydrogen’s unique physicochemical properties of hydrophobicity, neutrality, size, mass, etc. afford it with superior distribution properties allowing it to rapidly penetrate biomembranes (e.g. cell membranes, blood-brain, placental, and testis barrier) and reach subcellular compartments (e.g. mitochondria, nucleus, etc.) where it can exert its therapeutic effects [15].
Although various medical clinics in Japan use intravenous injection of hydrogen-rich saline, the most common methods are inhalation and drinking hydrogen-rich water. The pharmacokinetics of each method are still under investigation, but are dependent on dosage, route, and timing. An article published in Nature’s Scientific Reports [18] compared inhalation, injection and drinking with different hydrogen concentrations and found helpful insights for clinical use. Based on this and various studies, we briefly summarize the pharmacokinetics of inhalation and drinking.
INHALATION OF HYDROGEN
For inhalation, a 2-4% hydrogen gas mixture is common because it is below the flammability level; however, some studies use 66.7% H2 and 33.3% O2, which is nontoxic and effective, but flammable. Inhalation of hydrogen reaches a peak plasma level (i.e. equilibrium based on Henry’s Law) in about 30 min, and upon cessation of inhalation the return to baseline occurs in about 60 min.
DRINKING DISSOLVED HYDROGEN
The concentration/solubility of hydrogen in water at standard ambient temperature and pressure (SATP) is 0.8 mM or 1.6 ppm (1.6 mg/L). For reference, conventional water (e.g. tap, filtered, bottled, etc.) contains less than 0.0000002 ppm of H2, which is well below the therapeutic level (See Q&A 7-8). The concentration of 1.6 ppm is easily achieved by many methods, such as simply bubbling hydrogen gas into water. Because of molecular hydrogen’s low molar mass (i.e. 2.02 g/mol H2 vs. 176.12 g/mol vitamin C), there are more hydrogen molecules in a 1.6-mg dose of H2 than there are vitamin C molecules in a 100-mg dose of pure vitamin C (i.e. 1.6 mg H2 has 0.8 millimoles of H2 vs. 100 mg vitamin C has 0.57 millimoles of vitamin C).
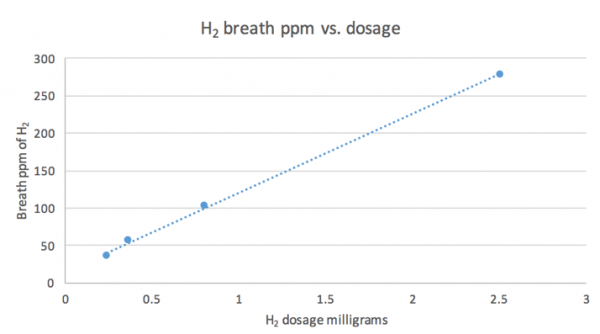
The half-life of hydrogen-rich water is shorter than other gaseous drinks (e.g. carbonated or oxygenated water), but therapeutic levels can remain for a sufficiently long enough time for easy consumption. Ingestion of hydrogen-rich water results in a peak rise in plasma and breath concentration in 5-15 min in a dose-dependent manner (see figure). The rise in breath hydrogen is an indication that hydrogen diffuses through the submucosa and enters systemic circulation where it is expelled out the lungs. This increase in blood and breath concentration returns to baseline in 45-90 min depending on the ingested dosage.
PHARMACODYNAMICS
Although a significant amount of research in cells, tissues, animals, humans and even plants have confirmed hydrogen’s effect in biological systems, the exact underlying molecular mechanisms and primary targets remain elusive [19].
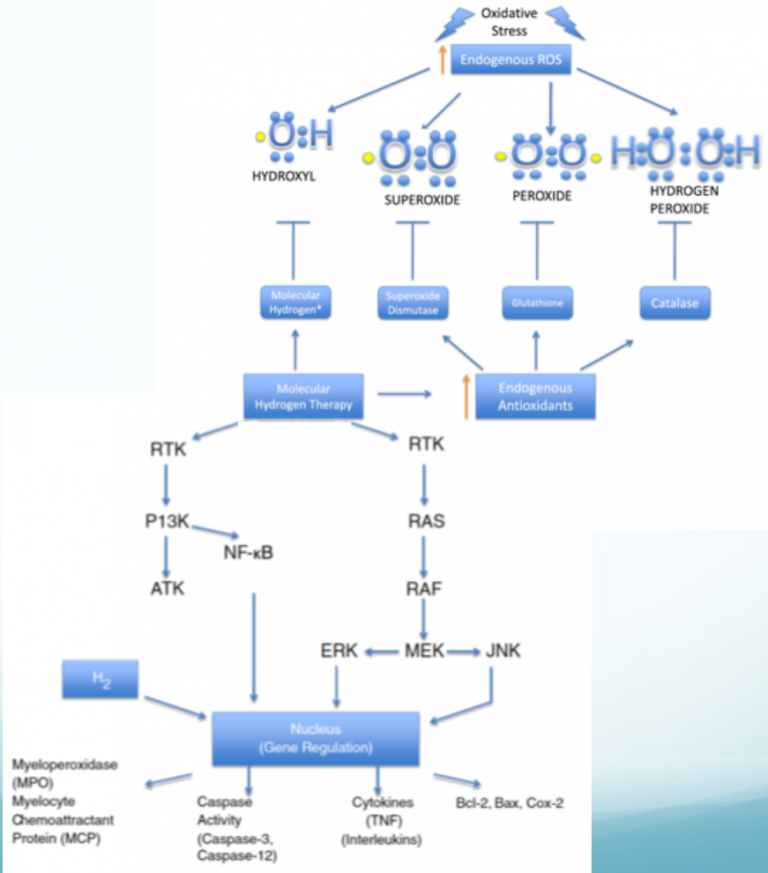
ANTIOXIDANT-LIKE EFFECT
It was initially suggested that the beneficial effect of hydrogen was due to an antioxidant as hydrogen selectively neutralized cytotoxic hydroxyl radicals [3] in vitro. However, although H2 reduces *OH radicals [20], as has been shown in various systems [3, 21, 22], it may not occur via direct scavenging, and it also cannot fully explain all the benefits of hydrogen [23]. For example, in a double-blinded placebo controlled trial in rheumatoid arthritis [24], hydrogen had a residual effect that continued improving the disease symptoms for four weeks after hydrogen administration was terminated [24]. Many cell studies also show that pre-treatment with hydrogen has marked beneficial effects even when the assault (e.g. toxin, radiation, injury, etc.) is administered long after all the hydrogen has dissipated out of the system [25-27]. Additionally, the rate constants of hydrogen against the hydroxyl radical are relatively slow (4.2 x 107 M-1 sec-1) [20], and the concentration of hydrogen at the cellular level is also quite low (micromolar levels), thus making it unlikely that H2 could effectively compete with the numerous other nucleophilic targets of the cell [28]. Lastly, if the mechanism were primarily associated with scavenging of hydroxyl radicals, then we should see a greater effect from inhalation compared to drinking, but this is not always the case [29, 30]. In short, we consider it inaccurate or at least incomplete to claim that the benefits of hydrogen are due to its acting directly as a powerful antioxidant. Indeed, hydrogen is selective because it is a very weak antioxidant and thus does not neutralize important ROSor disturb important biological signaling molecules. Nevertheless, a metabolic tracer study [31] using deuterium gas demonstrated that, under physiological conditions, deuterium gas is oxidized, and the oxidation rate of hydrogen increases with an increasing amount of oxidative stress [32], but the physicochemical mechanism for this may still not be direct radical scavenging [31]. However, not all studies show that hydrogen is oxidized via mammalian tissues [33], and it has also been reported that deuterium gas did not exert a therapeutic effect in the model studied whereas 1H did (unpublished data).
NRF2 PATHWAY
Unlike conventional antioxidants [34], hydrogen does have the ability to reduce excessive oxidative stress [23], but only under conditions where the cell is experiencing abnormally high levels of oxidative stress that would be harmful and not hormetic.
One mechanism that hydrogen uses to protect against oxidative damage is by the activation of the Nrf2-Keap1 system and subsequent induction of the antioxidant response element (ARE) pathway, which leads to the production of various cytoprotective proteins like glutathione, catalase, superoxide dismutase, glutathione peroxidase, heme-1 oxygenase, etc. [5, 35, 36]. In some disease models, the benefits of hydrogen are negated by using Nrf2 gene knockouts [37, 38], Nrf2 genetic silencing using iRNA [39], or pharmacologically blocking the Nrf2 pathway [40, 41]. Importantly, hydrogen only activates the Nrf2 pathway when there is an assault (e.g. toxin, injury, etc.) [40] as opposed to constituently acting as a promoter, which could be harmful [42, 43]. The method that hydrogen activates the Nrf2 pathway remains unclear [5].
CELL MODULATION
 Besides the potential scavenging of hydroxyl radicals and/or activation of the Nrf2 pathway, hydrogen may ameliorate oxidative stress via a cell modulating effect [5] and reduce the formation of free radicals [44], such as downregulating the NADPH oxidase system [45]. The various cell modulating effects of hydrogen are responsible for mediating the anti-inflammatory, anti-allergy, and anti-obesity effects of hydrogen. Hydrogen has been shown to downregulate pro-inflammatory cytokines (e.g. IL-1, IL-6, IL-8, etc.) [46], attenuate the activation of TNF-a [24], NF-?B [47], NFAT [30, 48], NLRP3 [49, 50], HMGB1 [51], and other inflammatory mediators [5]. Additionally, hydrogen has beneficial effects on obesity and metabolism by increasing the expression of FGF21 [52], PGC-1a [53], PPARa [53], and more. [54]. Additional 2nd messenger molecules or transcription factors affected by hydrogen include ghrelin [55], JNK-1 [45], ERK1/2 [56], PKC [57], GSK [58], TXNIP [49], STAT3 [59], ASK1 [60], MEK [61], SIRT1 [62], and many more. Over 200 biomolecules are altered by hydrogen administration including over 1000 gene expressions.
Besides the potential scavenging of hydroxyl radicals and/or activation of the Nrf2 pathway, hydrogen may ameliorate oxidative stress via a cell modulating effect [5] and reduce the formation of free radicals [44], such as downregulating the NADPH oxidase system [45]. The various cell modulating effects of hydrogen are responsible for mediating the anti-inflammatory, anti-allergy, and anti-obesity effects of hydrogen. Hydrogen has been shown to downregulate pro-inflammatory cytokines (e.g. IL-1, IL-6, IL-8, etc.) [46], attenuate the activation of TNF-a [24], NF-?B [47], NFAT [30, 48], NLRP3 [49, 50], HMGB1 [51], and other inflammatory mediators [5]. Additionally, hydrogen has beneficial effects on obesity and metabolism by increasing the expression of FGF21 [52], PGC-1a [53], PPARa [53], and more. [54]. Additional 2nd messenger molecules or transcription factors affected by hydrogen include ghrelin [55], JNK-1 [45], ERK1/2 [56], PKC [57], GSK [58], TXNIP [49], STAT3 [59], ASK1 [60], MEK [61], SIRT1 [62], and many more. Over 200 biomolecules are altered by hydrogen administration including over 1000 gene expressions.
 However, the primary targets and master regulators responsible for these changes are still elusive [46]. There are many feedback systems and loops to consider, which makes it difficult to determine if we are detecting the cause or the effect of hydrogen administration.
However, the primary targets and master regulators responsible for these changes are still elusive [46]. There are many feedback systems and loops to consider, which makes it difficult to determine if we are detecting the cause or the effect of hydrogen administration.
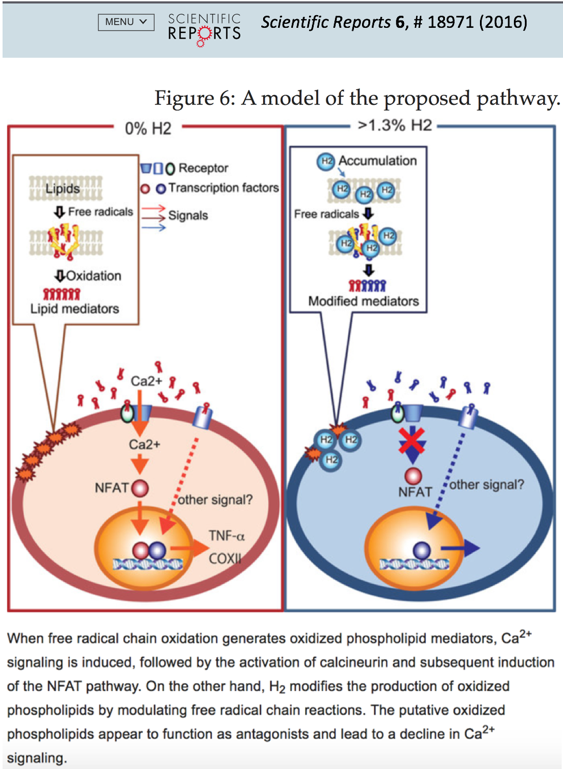
The exact mechanism of how hydrogen modulates signal transduction, gene expression, and protein phosphorylation is still being investigated [5]. A recent publication [63] in Scientific Reports provides good evidence to suggest that one of the mechanisms through which hydrogen accomplishes the various cell-modulating effects is by modifying lipid peroxidation in the cell membrane. In cultured cells, at biologically relevant concentrations, hydrogen suppressed the free radical chain reaction-dependent peroxidation and recovered Ca2+-induced gene expressions, as determined by comprehensive microarray analysis (see figure 6) [63].
SCIENTIFIC RECOGNITION OF HYDROGEN
Although the primary targets or exact biochemical mechanisms of hydrogen are still not fully understood, the therapeutic effect in cells, tissues, animals, humans and even plants [64] is becoming widely accepted due to the now over 500 peer-reviewed articles and the 1,600 researchers on the medical effects of hydrogen. The quality of the publications is also improving with an average impact factor (IF) of the journals publishing hydrogen is about 3. The table below shows a few of the studies published in the higher IF journals, which range from six to 27.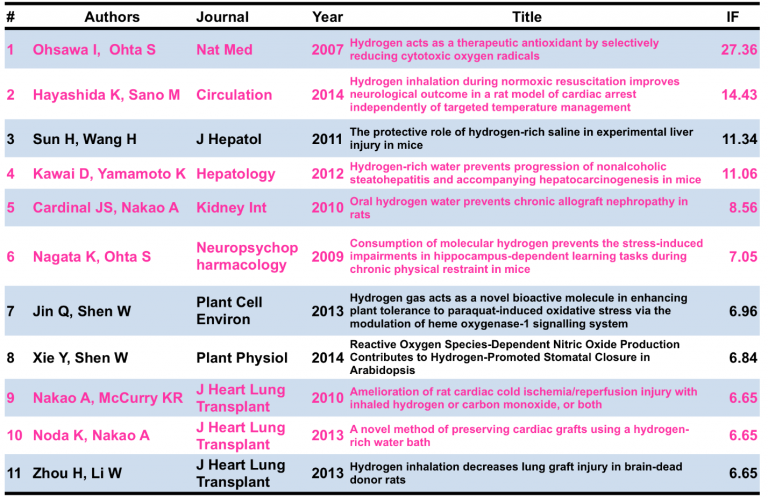
HYDROGEN AND IMMEDIATE MEDICAL APPLICATIONS
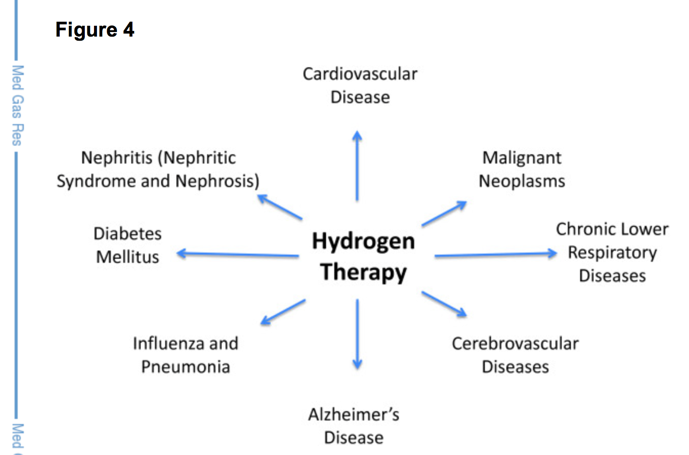 Hydrogen as a medical gas is also growing because it has immediate medical applications to help with many of the current health crises [65, 66]. Dixon and colleagues of Loma-Linda University reported that hydrogen has potential to help with the top 8/10 disease-causing fatalities as listed by the Centers of Disease Control [67]. Dr. Banks,from the VA/U of Washington, reported that ingestion of hydrogen-rich water was protective against neurodegenerative changes induced by traumatic brain injury in mice [68]. Their results show that hydrogen administration reduced brain edema, blocked pathological tau expression, and maintained ATP levels. This and other studies have profound effects for events where brain injury (e.g. concussion, chronic traumatic encephalopathy, etc.) is a common occurrence [69]. Although many people report dramatic effects of hydrogen therapy, from rapid pain and inflammation relief to normalization of glucose and cholesterol levels, other people may not notice any immediate or observable benefits. Hydrogen is not considered a powerful drug, and as mentioned only helps bring the cell/organ back to homeostasis without causing major perturbations. Perhaps some of the reported dramatic effects can be attributed to the placebo effect or other things, although some researchers have noted that some people are more sensitive to hydrogen and experience greater effects. More human studies are needed to answer these questions.
Hydrogen as a medical gas is also growing because it has immediate medical applications to help with many of the current health crises [65, 66]. Dixon and colleagues of Loma-Linda University reported that hydrogen has potential to help with the top 8/10 disease-causing fatalities as listed by the Centers of Disease Control [67]. Dr. Banks,from the VA/U of Washington, reported that ingestion of hydrogen-rich water was protective against neurodegenerative changes induced by traumatic brain injury in mice [68]. Their results show that hydrogen administration reduced brain edema, blocked pathological tau expression, and maintained ATP levels. This and other studies have profound effects for events where brain injury (e.g. concussion, chronic traumatic encephalopathy, etc.) is a common occurrence [69]. Although many people report dramatic effects of hydrogen therapy, from rapid pain and inflammation relief to normalization of glucose and cholesterol levels, other people may not notice any immediate or observable benefits. Hydrogen is not considered a powerful drug, and as mentioned only helps bring the cell/organ back to homeostasis without causing major perturbations. Perhaps some of the reported dramatic effects can be attributed to the placebo effect or other things, although some researchers have noted that some people are more sensitive to hydrogen and experience greater effects. More human studies are needed to answer these questions.
HUMAN RESEARCH
Although the research on hydrogen looks promising in the cell or animal models, more long-term clinical trials are required to confirm its efficacy in humans [70]. There are only a total of 40 human studies; few are in a double-blinded placebo controlled randomized fashion with sufficient subject numbers. A few of these clinical studies suggest that ingestion of hydrogen-rich water was beneficial for metabolic syndrome [71], diabetes [72], and hyperlipidemia [73, 74]. Another 1-year placebo-control clinical study suggested that hydrogen-rich water is beneficial for Parkinson’s disease [75], while other clinical studies suggest significant benefits for rheumatoid arthritis [24, 76], mitochondrial dysfunction [77], exercise performance [78], athletic recovery time [79], wound healing [80-82], reductions of oxidative stress from chronic hepatitis B [83], improvements to blood flow [84], and periodontitis [85], in dialysis [86, 87], and also the quality of life in patients receiving radiotherapy for tumors [88] and others [5].
There have been an additional 15+ human studies completed with promising results, which are in the process of manuscript preparation and publication through the peer-reviewed process. More human studies are required to determine proper dosage, timing, method of administration, and for which diseases, and potentially genotypes, hydrogen is most effective [7]. Hydrogen is still in its infancy, and more data is required before we can scientifically claim any real benefit, but the preliminary data is intriguing. The research on disease models, mechanisms of action, and clinical studies are particularly relevant because the high safety profile of molecular hydrogen make it a superior choice [89].
SAFETY
Hydrogen is naturally produced by intestinal flora upon digestion of fibers [90]. A study from the University of Florida and the Forsythe Institute of Boston, Massachusetts confirmed that hydrogen produced from bacteria exerted therapeutic effects [91]. They found that reconstitution of intestinal microbiota with H2-producing E. coli, but not H2-deficient mutant E. coli, was protective against Concanvalin A-induced hepatitis. Other studies also show that bacterially produced hydrogen from acarbose administration is therapeutic [92]. Perhaps this helps explain why a large clinical trial from the Journal of American Medical Association (JAMA) found significant reductions in cardiovascular events by those taking the hydrogen-producing acarbose drug [92, 93]. These studies not only suggest the therapeutic action of molecular hydrogen, but also demonstrate its high safety profile. Hydrogen is very natural to our bodies, as we are exposed to it on a daily basis as a result of normal bacterial metabolism [1]. Additionally, hydrogen gas has also been used in deep sea diving since the 1940s to prevent decompression sickness [94, 95]. Hundreds of human studies for deep sea diving have shown inhalation of hydrogen gas, at orders of magnitude greater than what is needed for therapeutic use, is well-tolerated by the body with no chronic toxic effects [96]. In some people, however, it is reported that hydrogen may result in loose stools [97], and in rare cases with diabetics, hypoglycemia [77], which is controlled by reducing the level of insulin administered. The hundreds of studies on hydrogen from bacterial production, deep sea diving, and recent medical applications have not revealed any direct noxious side effects of hydrogen administration at biologically therapeutic levels. Such a high safety profile may be considered paradoxical because chemotherapeutic agents that exert biological effects should have both beneficial and noxious effects depending on dosage, timing, location, duration, etc. However, such noxious effects have yet to be reported for hydrogen. However, perhaps the noxious effects are so transient and mild that they are masked by the beneficial effects, or are even what mediate the beneficial effects via hormetic pathways.
FUTURE DIRECTIONS
The goal of the Molecular Hydrogen Institute (MHI) is to help advance the research, education, and awareness of hydrogen as a therapeutic medical gas. It is uncommon to find a treatment that has both a high therapeutic potential and a high safety profile; hydrogen appears to fit this combination [23]. Some researchers have become interested in hydrogen due simply to its unforeseen ability to have a biological effect; with the realization that hydrogen is both safe and effective, a moral obligation develops to advance the research, education, and awareness of hydrogen as a medical gas. We welcome other biomedical researchers to join us in elucidating the in vitro molecular mechanisms of hydrogen, and to perform well-controlled clinical trials on hydrogen in order to understand the best dosage, timing, genotype, and method of hydrogen administration. With only a few hundred peer-reviewed articles and a couple thousand biomedical researchers, hydrogen research is still in its infancy. However, the preliminary studies suggest that molecular hydrogen is something that should be pursued, investigated, and elucidated for the potential benefit of disease prevention and treatment.
- George, J.F. and A. Agarwal, Hydrogen: another gas with therapeutic potential. Kidney International, 2010. 77(2): p. 85-87.
- Dole, M., F.R. Wilson, and W.P. Fife, Hyperbaric hydrogen therapy: a possible treatment for cancer. Science, 1975. 190(4210): p. 152-4.
- Ohsawa, I., et al., Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med, 2007. 13(6): p. 688-694.
- Ohta, S., Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther, 2014.
- Ichihara, M., et al., Beneficial biological effects and the underlying mechanisms of molecular hydrogen – comprehensive review of 321 original articles. Med Gas Res, 2015. 5: p. 12.
- Fandrey, J., Rounding up the usual suspects in O2 sensing: CO, NO, and H2S! Sci Signal, 2015. 8(373): p. fs10.
- Zhai, X., et al., Review and prospect of the biomedical effects of hydrogen. Med Gas Res, 2014. 4(1): p. 19.
- Ohta, S., Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochimica et Biophysica Acta, 2012. 1820(5): p. 586-94.
- Martin, W. and M. Muller, The hydrogen hypothesis for the first eukaryote. Nature, 1998. 392(6671): p. 37-41.
- Chen, O., Z.-h. Y., and C. Li., Meeting report: Second Hydrogen Molecule Biomedical Symposium in Beijing, China. Medical Gas Research, 2016. 6(1): p. 57. (See LeBaron)
- Hayashida, K., et al., Hydrogen Inhalation During Normoxic Resuscitation Improves Neurological Outcome in a Rat Model of Cardiac Arrest, Independent of Targeted Temperature Management. Circulation, 2014.
- Kawai, D., et al., Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology, 2012. 56(3): p. 912-21.
- Nakayama, M., et al., Less-oxidative hemodialysis solution rendered by cathode-side application of electrolyzed water. Hemodial Int, 2007. 11(3): p. 322-7.
- Sun, H., et al., The protective role of hydrogen-rich saline in experimental liver injury in mice. Journal of Hepatology, 2011. 54(3): p. 471-80.
- Qian, L., J. Shen, and X. Sun, Methods of Hydrogen Application. Hydrogen Molecular Biology and Medicine. 2015: Springer Netherlands.
- Nishimura, N., et al., Pectin and high-amylose maize starch increase caecal hydrogen production and relieve hepatic ischaemia-reperfusion injury in rats. Br J Nutr, 2012. 107(4): p. 485-92.
- Senn, N., RECTAL INSUFFLATION OF HYDROGEN GAS AN INFALLIBLE TEST IN THE DIAGNOSIS OF VISCERAL INJURY OF THE GASTRO INTESTINAL CANAL IN PENETRATING WOUNDS OF THE ABDOMEN. Read in the Section on Surgery, at the Thirty-ninth Annual Meeting of the American Medical Association, May, 9, 1888, and illuistrated by three experiments on dogs.”. JAMA: Journal of the American Medical Association, 1888. 10(25): p. 767-777.
- Liu, C., et al., Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep, 2014. 4: p. 5485.
- Ohta, S., Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des, 2011. 17(22): p. 2241-52.
- Buxton, G.V., et al., Critical view of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•OH-) in aqueous solution. J Phys Chem Ref Data, 1988. 17: p. 513-886.
- Igarashi, T., et al., Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci Rep, 2016. 6: p. 31190.
- Terasaki, Y., et al., Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. American Journal of Physiology – Lung Cellular and Molecular Physiology, 2011. 301(4): p. L415-26.
- Ohta, S., Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol, 2015. 555: p. 289-317.
- Ishibashi, T., et al., Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int Immunopharmacol, 2014. 21(2): p. 468-473.
- Zhang, J.Y., et al., Protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. World J Gastroenterol, 2014. 20(6): p. 1614-22.
- Gu, H., et al., Pretreatment with hydrogen-rich saline reduces the damage caused by glycerol-induced rhabdomyolysis and acute kidney injury in rats. J Surg Res, 2014. 188(1): p. 243-9.
- Kawasaki, H., J.J. Guan, and K. Tamama, Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochemical and Biophysical Research Communications, 2010. 397(3): p. 608-613.
- Wood, K.C. and M.T. Gladwin, The hydrogen highway to reperfusion therapy. Nat Med, 2007. 13(6): p. 673-674.
- Ito, M., et al., Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med Gas Res, 2012. 2(1): p. 15.
- Sobue, S., et al., Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol Cell Biochem, 2015. 403(1-2): p. 231-41.
- Hyspler, R., et al., The Evaluation and Quantitation of Dihydrogen Metabolism Using Deuterium Isotope in Rats. PLoS One, 2015. 10(6): p. e0130687.
- Shimouchi, A., et al., Molecular hydrogen consumption in the human body during the inhalation of hydrogen gas. Adv Exp Med Biol, 2013. 789: p. 315-21.
- Kayar, S.R., et al., Hydrogen Gas Is Not Oxidized by Mammalian-Tissues under Hyperbaric Conditions. Undersea & Hyperbaric Medicine, 1994. 21(3): p. 265-275.
- McCall, M.R. and B. Frei, Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med, 1999. 26(7-8): p. 1034-53.
- Yu, J., et al., Molecular hydrogen attenuates hypoxia/reoxygenation injury of intrahepatic cholangiocytes by activating Nrf2 expression. Toxicol Lett, 2015. 238(3): p. 11-19.
- Diao, M., et al., Hydrogen Gas Inhalation Attenuates Seawater Instillation-Induced Acute Lung Injury via the Nrf2 Pathway in Rabbits. Inflammation, 2016.
- Xie, K., et al., Nrf2 is critical in the protective role of hydrogen gas against murine polymicrobial sepsis. British Journal of Anaesthesia, 2012. 108(3): p. 538-539.
- Kawamura, T., et al., Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol, 2013. 304(10): p. L646-56.
- Xie, Q., et al., Hydrogen gas protects against serum and glucose deprivation induced myocardial injury in H9c2 cells through activation of the NFE2 related factor 2/heme oxygenase 1 signaling pathway. Mol Med Rep, 2014. 10(2): p. 1143-9.
- Hara, F., et al., Molecular Hydrogen Alleviates Cellular Senescence in Endothelial Cells. Circ J, 2016.
- Chen, H., et al., Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol, 2015. 28(1): p. 643-54.
- Wakabayashi, N., et al., Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet, 2003. 35(3): p. 238-45.
- Rajasekaran, N.S., et al., Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal, 2011. 14(6): p. 957-71.
- Sato, Y., et al., Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun, 2008. 375(3): p. 346-350.
- Itoh, T., et al., Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun, 2009. 389(4): p. 651-6.
- Ohno, K., M. Ito, and M. Ichihara, Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxidative Medicine and Cellular Longevity, 2012. 2012: p. 353152.
- Wang, C., et al., Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neuroscience Letters, 2011. 491(2): p. 127-32.
- Kishimoto, Y., et al., Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J Thorac Cardiovasc Surg, 2015. 150(3): p. 645-654 e3.
- Ren, J.D., et al., Hydrogen-rich saline inhibits NLRP3 inflammasome activation and attenuates experimental acute pancreatitis in mice. Mediators Inflamm, 2014. 2014: p. 930894.
- Shao, A., et al., Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-kappaB Pathway and NLRP3 Inflammasome. Mol Neurobiol, 2015.
- Xie, K.L., et al., [Effects of hydrogen gas inhalation on serum high mobility group box 1 levels in severe septic mice]. Zhejiang Da Xue Xue Bao Yi Xue Ban, 2010. 39(5): p. 454-7.
- Kamimura, N., et al., Molecular Hydrogen Improves Obesity and Diabetes by Inducing Hepatic FGF21 and Stimulating Energy Metabolism in db/db Mice. Obesity, 2011.
- Kamimura, N., et al., Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1 [alpha] to enhance fatty acid metabolism. NPJ Aging and Mechanisms of Disease, 2016. 2: p. 16008.
- Zhang, J.Y., et al., A Review of Hydrogen as a New Medical Therapy. Hepato-Gastroenterology, 2012. 59(116): p. 1026-1032.
- Matsumoto, A., et al., Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep, 2013. 3: p. 3273.
- Sun, Y., et al., Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos Int, 2013. 24(3): p. 969-78.
- Amitani, H., et al., Hydrogen Improves Glycemic Control in Type1 Diabetic Animal Model by Promoting Glucose Uptake into Skeletal Muscle. PLoS One, 2013. 8(1).
- Hong, Y., et al., Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3beta signaling pathway. PLoS One, 2014. 9(4): p. e96212.
- Li, F.Y., et al., Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol, 2013. 61: p. 248-54.
- Itoh, T., et al., Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biochemical and Biophysical Research Communications, 2011. 411(1): p. 143-9.
- Cardinal, J.S., et al., Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney International, 2010. 77(2): p. 101-9.
- Lin, C.L., et al., Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact, 2015. 240: p. 12-21.
- Iuchi, K., et al., Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep, 2016. 6: p. 18971.
- Jin, Q., et al., Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell and Environment, 2013. 36(5): p. 956-69.
- Zheng, Y. and D. Zhu, Molecular Hydrogen Therapy Ameliorates Organ Damage Induced by Sepsis. Oxid Med Cell Longev, 2016. 2016: p. 5806057.
- Nicolson, G.L., et al., Clinical Effects of Hydrogen Administration: From Animal and Human Diseases to Exercise Medicine. International Journal of Clinical Medicine, 2016. 7(1).
- Dixon, B.J., J. Tang, and J.H. Zhang, The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res, 2013. 3(1): p. 10.
- Dohi, K., et al., Molecular Hydrogen in Drinking Water Protects against Neurodegenerative Changes Induced by Traumatic Brain Injury. PLoS One, 2014. 9(9): p. e108034.
- Xie, F. and X. Ma, Molecular Hydrogen and its Potential Application in Therapy of Brain Disorders. Brain Disord Ther, 2014: p. 2.
- Chen, X., X. Sun, and S. Ohta, Future Directions in Hydrogen Studies. Hydrogen Molecular Biology and Medicine. 2015: Springer Netherlands.
- Nakao, A., et al., Effectiveness of Hydrogen Rich Water on Antioxidant Status of Subjects with Potential Metabolic Syndrome-An Open Label Pilot Study. Journal of Clinical Biochemistry and Nutrition, 2010. 46(2): p. 140-149.
- Kajiyama, S., et al., Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutrition Research, 2008. 28: p. 137–143.
- Song, G., et al., Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. Journal of Lipid Research, 2013. 54(7): p. 1884-93.
- Zong, C., et al., Cigarette smoke exposure impairs reverse cholesterol transport which can be minimized by treatment of hydrogen-saturated saline. Lipids Health Dis, 2015. 14: p. 159.
- Yoritaka, A., et al., Pilot study of H(2) therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Movement Disorders, 2013.
- Ishibashi, T., et al., Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Medical Gas Research, 2012. 2(1): p. 27.
- Ito, M., et al., Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Medical Gas Research, 2011. 1(1): p. 24.
- Aoki, K., et al., Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Medical Gas Research, 2012. 2(1): p. 12.
- Ostojic, S.M., et al., Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad Med, 2014. 126(5): p. 187-95.
- Ishibashi, T., et al., Improvement of psoriasis-associated arthritis and skin lesions by treatment with molecular hydrogen: A report of three cases. Mol Med Rep, 2015. 12(2): p. 2757-64.
- Ono, H., et al., Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Medical Gas Research, 2012. 2(1): p. 14.
- Li, Q., et al., Hydrogen water intake via tube-feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med Gas Res, 2013. 3(1): p. 20.
- Xia, C., et al., Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clin Transl Sci, 2013. 6(5): p. 372-5.
- Sakai, T., et al., Consumption of water containing over 3.5 mg of dissolved hydrogen could improve vascular endothelial function. Vasc Health Risk Manag, 2014. 10: p. 591-7.
- Azuma, T., et al., Drinking Hydrogen-Rich Water Has Additive Effects on Non-Surgical Periodontal Treatment of Improving Periodontitis: A Pilot Study. Antioxidants 2015. 4(3): p. 513-522.
- Nakayama, M., et al., Biological Effects of Electrolyzed Water in Hemodialysis. Nephron Clinical Practice, 2009. 112(1): p. C9-C15.
- Huang, K.C., et al., Electrolysed-reduced water dialysate improves T-cell damage in end-stage renal disease patients with chronic haemodialysis. Nephrology Dialysis Transplantation, 2010. 25(8): p. 2730-2737.
- Kang, K.-M., et al., Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Medical Gas Research, 2011. 1: p. 11.
- Tao, Y., et al., The potential utilizations of hydrogen as a promising therapeutic strategy against ocular diseases. Ther Clin Risk Manag, 2016. 12: p. 799-806.
- Eastwood, M.A., The physiological effect of dietary fiber: an update. Annu Rev Nutr, 1992. 12: p. 19-35.
- Kajiya, M., et al., Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun, 2009. 386(2): p. 316-21.
- Zhang, D.Q., J.H. Zhu, and W.C. Chen, Acarbose: a new option in the treatment of ulcerative colitis by increasing hydrogen production. Afr J Tradit Complement Altern Med, 2012. 10(1): p. 166-9.
- Chiasson, J.L., et al., Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA, 2003. 290(4): p. 486-94.
- Case, E.M. and J.B. Haldane, Human physiology under high pressure: I. Effects of Nitrogen, Carbon Dioxide, and Cold. J Hyg (Lond), 1941. 41(3): p. 225-49.
- Dougherty, J.H., Jr., Use of H2 as an inert gas during diving: pulmonary function during H2-O2 breathing at 7.06 ATA. Aviat Space Environ Med, 1976. 47(6): p. 618-26.
- Friess, S.L., W.V. Hudak, and R.D. Boyer, Toxicology of hydrogen-containing diving environments. I. Antagonism of acute CO2 effects in the rat by elevated partial pressures of H2 gas. Toxicol Appl Pharmacol, 1978. 46(3): p. 717-25.
- Nagatani, K., et al., Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med Gas Res, 2013. 3: p. 13.
A Call Back






